LYT-310 demonstrated three to fourfold increase in oral bioavailability of CBD compared to unmodified CBD in a preclinical model
Oral dosing and potential for improved tolerability could expand therapeutic application of CBD across a wider range of age groups and indications, including both rare and more common forms of epilepsy and other central nervous system disorders
Further proof of Glyph platform’s ability to enable oral administration of certain small molecules with otherwise limited oral bioavailability
PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) ("PureTech" or the "Company"), a clinical-stage biotherapeutics company dedicated to changing the treatment paradigm for devastating diseases, today announced the nomination of a new therapeutic candidate, LYT-310, which is an oral cannabidiol (CBD) prodrug and the second therapeutic candidate developed from PureTech’s Glyph™ platform to be advanced toward the clinic. Clinical studies of LYT-310 are expected to begin in Q4 of 2023.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221130005402/en/
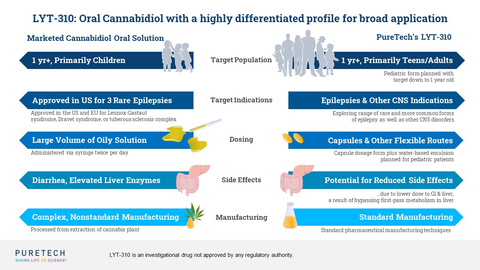
PureTech announced the nomination of a new therapeutic candidate, LYT-310, which is an oral cannabidiol (CBD) prodrug and the second therapeutic candidate developed from PureTech’s Glyph™ platform to be advanced toward the clinic. Oral capsule dosing and the potential for improved tolerability could expand the therapeutic application of CBD across a wider range of age groups and indications, including both rare and more common forms of epilepsy and other central nervous system disorders. (Graphic: Business Wire)
A CBD-based product has received regulatory approval in the United States and Europe to treat seizures resulting from certain rare conditions, but it requires a large volume of a sesame oil-based formulation, which limits its use in broader indications and age groups. PureTech’s LYT-310 is designed to greatly expand the therapeutic application and potential of CBD by:
- enabling oral administration in a capsule;
- expanding the use of CBD into a broad range of therapeutic areas and patient populations (such as adolescents and adults) where higher doses are required to achieve a therapeutic effect;
- potentially improving safety and reducing gastrointestinal (GI) tract side effects that are associated with the currently approved CBD-based treatment by reducing GI and liver exposure; and
- allowing for a readily scalable, consistent product in a cost-effective manner.
“The nomination of LYT-310 is an exciting expansion of PureTech’s Glyph technology,” said Daniel Bonner, Ph.D., Vice President at PureTech Health. “The data generated to date with LYT-310 further demonstrate our ability to apply the Glyph technology to an array of molecules to enable or greatly enhance oral bioavailability. This approach allows us to unlock the therapeutic potential of a range of molecules with validated efficacy whose development has been limited by first pass metabolism by the liver.”
In multiple preclinical models, including large animal and non-human primate, LYT-310 has demonstrated a three to fourfold increase in oral exposure vs. unmodified CBD in a fasted state. This has the potential to translate into improved safety and reduced side effects. Lymphatic transport has also been confirmed in preclinical models, with up to 30% of LYT-310 entering the lymphatics, compared to 5% for unmodified CBD – which further supports the novel Glyph mechanism of enhancing bioavailability.
The first candidate from the Glyph technology platform, LYT-300 (oral allopregnanolone), is currently being evaluated in a multi-part Phase 1 trial designed to demonstrate oral bioavailability, evaluate safety and tolerability across a range of doses, and to inform dose selection moving forward. The first objective was achieved earlier this year, and LYT-300 demonstrated oral bioavailability of allopregnanolone approximately ninefold greater than that of orally administered allopregnanolone, based on previously published data. The Phase 1 clinical trial is expected to be completed by the end of 2022, and – based on the data – a Phase 1b/2a clinical trial is planned to initiate in 2023.
About the GlyphTM Platform
Glyph is PureTech’s synthetic lymphatic-targeting chemistry platform which is designed to employ the lymphatic system’s natural lipid absorption and transport process to enable the oral administration of certain therapeutics. Glyph reversibly links a drug to a dietary fat molecule, creating a novel prodrug. The linked fat molecule re-routes the drug’s normal path to the systemic circulation, bypassing the liver and instead moving from the gut into the lymphatic vessels that normally process dietary fats. PureTech believes this technology has the potential to (1) enable direct modulation of the immune system via drug targets present in mesenteric lymph nodes and (2) provide a broadly applicable means of enhancing the bioavailability of certain orally administered drugs that would otherwise be limited by first-pass liver metabolism. PureTech is accelerating development of a Glyph portfolio that leverages validated efficacy, prioritizing highly characterized drugs to evaluate the ability of the Glyph technology to improve oral bioavailability or lymphatic targeting. PureTech’s lead Glyph therapeutic candidate, LYT-300 (oral allopregnanolone), is currently being evaluated in a multi-part Phase 1 clinical trial that is expected to read out by the end of 2022. A second therapeutic candidate, LYT-310 (oral cannabidiol), is expected to enter the clinic in Q4 of 2023. PureTech has a robust intellectual property portfolio that includes licensed patents as well as wholly owned patents, covering the Glyph technology platform, which is based on the pioneering research of Christopher Porter, Ph.D., and his research group at the Monash Institute of Pharmaceutical Sciences at Monash University. The Porter Research Group and collaborators have published research in Nature Medicine, Angewandte Chemie and the Journal of Controlled Release supporting the Glyph platform’s ability to directly target the lymphatic system with a variety of therapies.
About PureTech Health
PureTech is a biotherapeutics company dedicated to changing the treatment paradigm for devastating diseases. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech's Founded Entities, is comprised of 28 therapeutics and therapeutic candidates, including two (Plenity® and EndeavorRx®) that have received both U.S. FDA clearance and European marketing authorization and a third (KarXT) that will soon be filed for FDA approval, as of the most recent update by the Company. All of the underlying programs and platforms that resulted in this pipeline of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on unique insights in immunology and drug development.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact, including without limitation those statements that relate to the potential for improved tolerability associated with LYT-310 as compared to unmodified CBD, that such potential improved tolerability and oral dosing could expand the therapeutic application of CBD across a wider range of age groups and indications, our expectations regarding the Glyph™ technology platform including the potential for new treatment applications, the applicability of preclinical results to human subjects, the timing of clinical trials associated with LYT-300 , our product candidates and approach towards addressing major diseases, and our future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks, uncertainties and other important factors that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks, uncertainties and other important factors described under the caption “Risk Factors” in our Annual Report on Form 20-F for the year ended December 31, 2021 filed with the SEC and in our other regulatory filings. These forward-looking statements are based on assumptions regarding the present and future business strategies of the Company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, we disclaim any obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221130005402/en/
Contacts
PureTech
Public Relations
publicrelations@puretechhealth.com
Investor Relations
IR@puretechhealth.com
Media
Nichole Sarkis
+1 774 278 8273
nichole@tenbridgecommunications.com





