
As the year 2022 came to a close, Sofwave Medical announced that its revolutionary SUPERBTM technology received clearance from the FDA for short-term improvement in the appearance of cellulite. The emerging leader in energy-based non-invasive medical devices for aesthetic treatments is recognized for its cutting-edge aesthetic technology that delivers desired results quickly.
An estimated 85% of women are familiar with cellulite, dimply skin that is most prevalent in women than men. Cellulite commonly appears on the thighs but is also present in other parts of the body, like the stomach and hips. The tightly structured fat cells cause the outer skin to get unsightly dimples, a source of insecurity for many people. For a long time, people have tried many cellulite treatments advertised to be effective but often leave people disappointed. Over the years, cellulite reduction options have increased from creams to massages, laser treatments, radiofrequency treatments, and more.

Anti-cellulite treatments are among the most searched aesthetic treatment options in medical aesthetics. The cellulite treatment market has been reporting record growth rates each year as more people seek alternative non-invasive options that are safe and effective. Sofwave SUPERBTM technology, a disruptive innovative solution, is designed to revolutionize the growing aesthetic technology market.
In 2020, Sofwave Medical unveiled its Synchronous Ultrasound Parallel Beam (SUPERBTM) technology, created for treating wrinkles and lax skin. Since its launch, the technology has helped many people access non-invasive aesthetic treatments. The technology has also been highly reviewed by physicians across the globe, quickly establishing SUPERBTM technology as a frontrunner in cosmetic procedures.
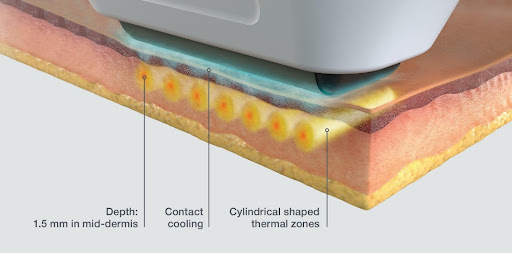
The innovative SUPERBTM technology emits synchronous beams of ultrasound that heat skin tissue to achieve positive results. SUPERBTM technology targets the mid-dermis, making the treatment more effective, with patients achieving results in half the time other technologies take. Sofwave treatments are ideal for all skin types since the microscopic and bulk mechanical properties of tissue determine the ultrasound absorption.
SUPERBTM Technology received FDA clearance for additional indications in 2021. The technology was cleared for lifting eyebrows, lifting lax submental tissue, and neck tissue. The aesthetic treatment technology has since received clearance for non-invasive dermatological aesthetic treatment for facial lines and wrinkle reduction. In 2022, it was cleared for short-term improvement in the appearance of cellulite.

Besides guaranteeing stellar results, the state-of-the-art aesthetic technology is also renowned for its short and convenient treatment times. Treatment sessions are typically 30-45 minutes with no post-treatment discomfort. The Sofwave SofcoolTM cooling mechanism helps protect the skin from burning as the ultrasound energy penetrates the skin surface to reach the mid-dermis.
As Sofwave SUPERBTM technology becomes a go-to for short-term cellulite treatment, many physicians across the globe have termed the technology a breakthrough for the aesthetic industry with smart yet simple, safe, and practical solutions.
To learn more about the Sofwave SUPERBTM technology and its innovative aesthetic solutions, visit Sofwave.com.
Media Contact
Company Name: Sofwave Medical
Contact Person: Bella
Email: Send Email
Country: Israel
Website: https://sofwave.com/news/sofwave-medical-announces-fda-clearance-of-superb-technology-for-cellulite/




