- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
- Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
- D. Boral Analyst Report on NRXP $34 Price Target.
- Abbreviated New Drug Application for KETAFREE™ Received Supportive Correspondence from FDA and Expects Q2 2026 GDUFA Date.
- Three Revenue-Generating Facilities in Florida and Expects Six More by Year-End for HOPE Subsidiary Clinics.
- Secured Operating Capital for Drug Development Through July 2026. Expecting Increased Revenue from Clinical Operations.
- Received FDA Grant of Fast Track Designation for NRX-100 in Treatment of Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
- New Real-World Data Support D-Cycloserine (NRX-101 Active Ingredient) in Doubling Effectiveness of Transcranial Magnetic Stimulation.
- Manufactured Multiple Commercial Lots of NRX-100 and KETAFREE™ with Ongoing Stability Data Supporting a Room Temperature Shelf Life of Three Years.
- First-in-Florida Initiation of One Day (ONE-D) Depression Treatment in Partnership with Ampa Health.
- ONE-D is the First Reported Protocol to Achieve Remission from Treatment-Resistant Depression via Single Day of Treatment, Using an FDA-Cleared Device.
- Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
- Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
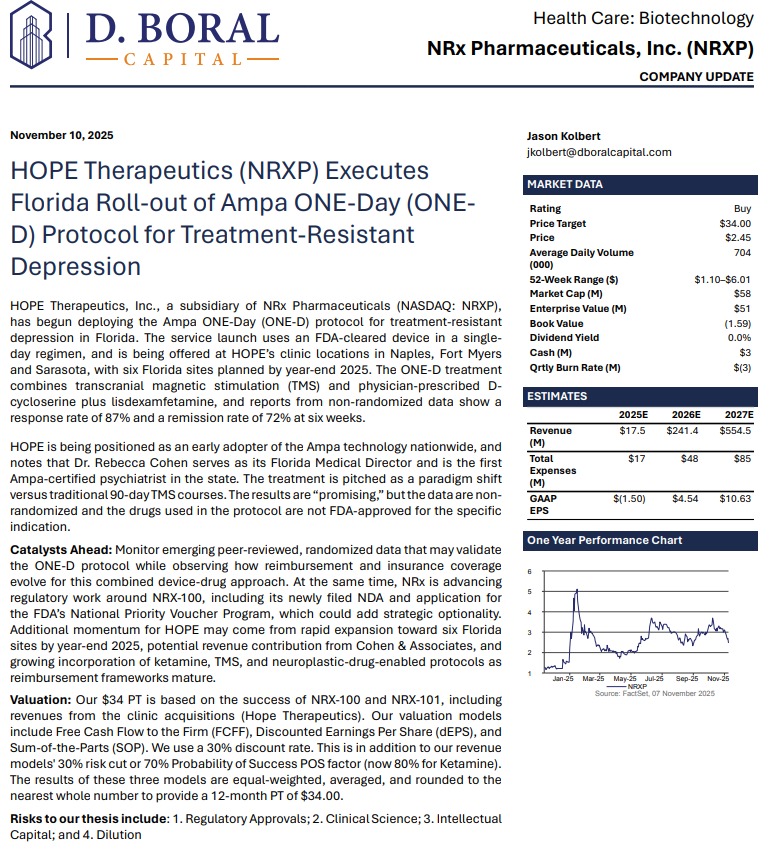
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
D. Boral has issued an Analyst Report on NRXP with a Buy and $34 Price Target. The full report may be accessed at this direct link: https://www.nrxpharma.com/wp-content/uploads/2025/11/HOPE-Therapeutics-NRXP-Executes-Florida-Roll-out-of-Ampa-O.pdf
Third Quarter 2025 Corporate Update
On November 17th NRXP announced financial results for the quarter ended September 30, 2025, and provided a corporate update.
Dr. Jonathan Javitt, CEO of NRXP stated, “In 2025 we have advanced each of our corporate objectives and entered into revenue-generating activity for the first time. For NRX-100 in suicidal depression, we received an expanded Fast Track designation, opened an Expanded Access program and enhanced our regulatory package. Additionally, FDA granted our Suitability Petition for a single patient, preservative free ketamine strength and we have received validation that our ANDA filing is on track with no major deficiencies. In parallel, the Real World Data demonstrating a doubling of antidepressant and antisuicidal effect of Transcranial Magnetic Stimulation (TMS) when D-cycloserine is added creates a new and significant indication for NRX-101 that has potential for approval, if confirmed in an additional phase 3 trial. For HOPE, we continue to execute on building our delivery platform of three active facilities in Florida, with three more planned by year-end. Dr. Rebecca Cohen and LTC Charles Paul, RN (US Army, Ret.) are assembling a network of best-in-class interventional psychiatrists to meet the needs of people, including active duty military, first responders, and veterans, across Florida and beyond.”

Key Research and Development and Corporate Activities:NRXP is pursuing two paths to market for NRX-100: a distinct innovative pathway via a New Drug Application (NDA) under FDA Fast Track designation to develop NRX-100 for suicidal ideation in depression, including bipolar depression, and a generic pathway via an Abbreviated New Drug Application (ANDA) to supply a preservative-free ketamine (KETAFREE™) into the existing ketamine market.
Recent correspondence from the FDA suggests that the latter pathway is on track for a Q2 2026 GDUFA date. The current generic ketamine market is estimated at approximately $750 million. By contrast, the innovative ketamine-based product SPRAVATO® is expected to generate over $1.6 billion in 2025 sales, although its labeling states that effectiveness in preventing suicide or reducing suicidal ideation has not been demonstrated. This highlights a differentiated opportunity for NRXP NRX-100, specifically in suicidal ideation. A New Drug Application (“NDA”) for NRX-100 for suicidal depression, originally initiated during the fourth quarter of 2024, is expected to be completed in the fourth quarter 2025 with the addition of Real World Efficacy Data drawn from more than 60,000 patients treated for depression with intravenous ketamine compared to 6,000 patients treated with intranasal S-ketamine to be submitted as part of the NDA. An interim analysis drawn from the first 20,000 patients suggests that IV ketamine may have a more rapid onset of action and larger magnitude of effect than nasal S-ketamine. The Company has applied to receive a Commissioner’s National Priority Voucher (CNPV), which could significantly reduce review time.
The Abbreviated New Drug Application (“ANDA”) for NRX-100 was filed, with Priority Review requested, during the third quarter of 2025. After meeting with the FDA in August 2025, the Company re-filed the ANDA, following FDA notification of approval of a Suitability Petition for NRx’s proposed strength of preservative-free ketamine, KETAFREE™. On November 6, 2025, NRXP received a communication from FDA in which no significant deficiencies were identified in the revised filing. NRXP has additionally submitted a Citizen Petition seeking to have benzethonium chloride, a toxic preservative, removed from all commercial presentations of ketamine.
NRXP has now manufactured multiple commercial lots of NRX-100 and KETAFREE™ with ongoing stability data supporting a room temperature shelf life of three years.
Third Quarter 2025 Financial Results
NRXP third quarter 2025 financial results were released on November 17th via public disclosure media and on the Company's website at https://ir.nrxpharma.com/. NRXP management also hosted a conference call on the same day. A live webcast of the conference call will be available on the NRXP website at https://ir.nrxpharma.com/events.
First-in-Florida Initiation of One Day (ONE-D) Depression Treatment in Partnership with Ampa Health
On November 10th NRXP announced initiation of patient care with for treatment-resistant depression with the Ampa one day (ONE-D) protocol. NRXP is the first to deploy the Ampa technology in Florida and one of the first deployments nationwide. The Ampa device differs from other Transcranial Magnetic Stimulation (TMS) treatments in that the peer-reviewed literature has reported a high rate of success (87% response and 72% remission) in nonrandomized trials when a single day of TMS treatment is combined with physician-prescribed D-cycloserine and lisdexamfetamine (note that neither of the drugs reported in the published results is FDA-approved for the stated indication).
The Ampa device is initially deployed at multiple NRXP HOPE clinic locations in Florida including Sarasota, Naples and Fort Myers, under the direction of Rebecca Cohen, MD, HOPE’s Medical Director, with six locations in Florida planned by year-end 2025. The ONE-D protocol offers a new treatment paradigm to patients with severe depression who previously were required to undergo 90 days of TMS. D-cycloserine is an active component of the NRXP NRX-101, a Breakthrough Therapy designated investigational drug that is available under an expanded access protocol (www.clinicaltrials.gov NCT05779267) and Federal and State Right to Try regulations.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: Send Email
Phone: 484 254 6134
Address:1201 Orange Street Suite 600
City: Miami
State: Florida
Country: United States
Website: https://www.nrxpharma.com/






